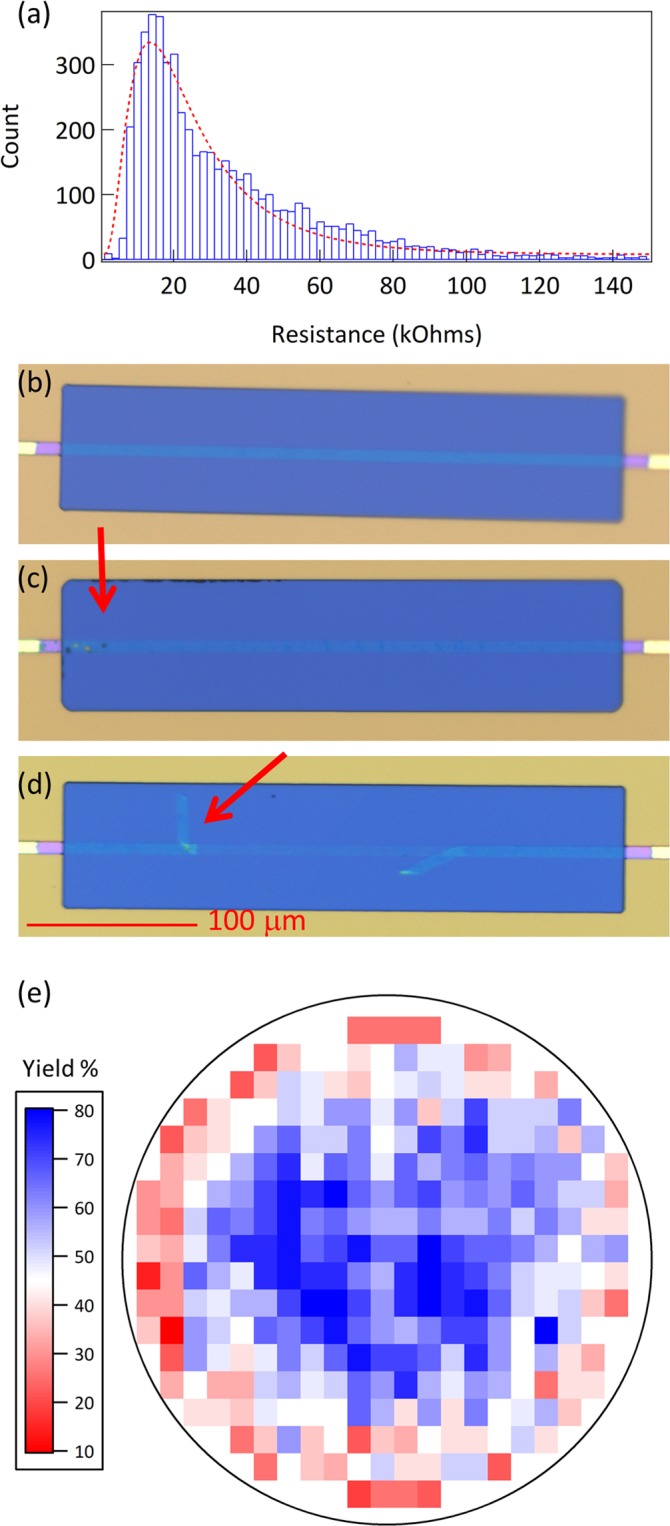
The discovery of new medicines has become increasingly more challenging and requires significant collaboration between pharma, biotech, academia, and technology to be successful.
These partnerships necessitate the streamlined exchange of samples while adhering to the increasingly complex set of legal and proprietary restrictions, government legislation, and ethical considerations associated with samples. There is a significant volume of literature published on clinical sample compliance but little describing compliance aspects of discovery sample management.
This paper describes some of the key compliance activities and challenges and shares GlaxoSmithKline’s experiences and current practices.
Synthetic biology is an established but ever-growing interdisciplinary field of research currently revolutionizing biomedicine studies and the biotech industry.
The engineering of synthetic circuitry in bacterial, yeast, and animal systems prompted considerable advances for the understanding and manipulation of genetic and metabolic networks; however, their implementation in the plant field lags behind.
Here, we review theoretical-experimental approaches to the engineering of synthetic chemical- and light-regulated (optogenetic) switches for the targeted interrogation and control of cellular processes, including existing applications in the plant field.
We highlight the strategies for the modular assembly of genetic parts into synthetic circuits of different complexity, ranging from Boolean logic gates and oscillatory devices up to semi- and fully synthetic open- and closed-loop molecular and cellular circuits.
Finally, we explore potential applications of these approaches for the engineering of novel functionalities in plants, including understanding complex signaling networks, improving crop productivity, and the production of biopharmaceuticals.
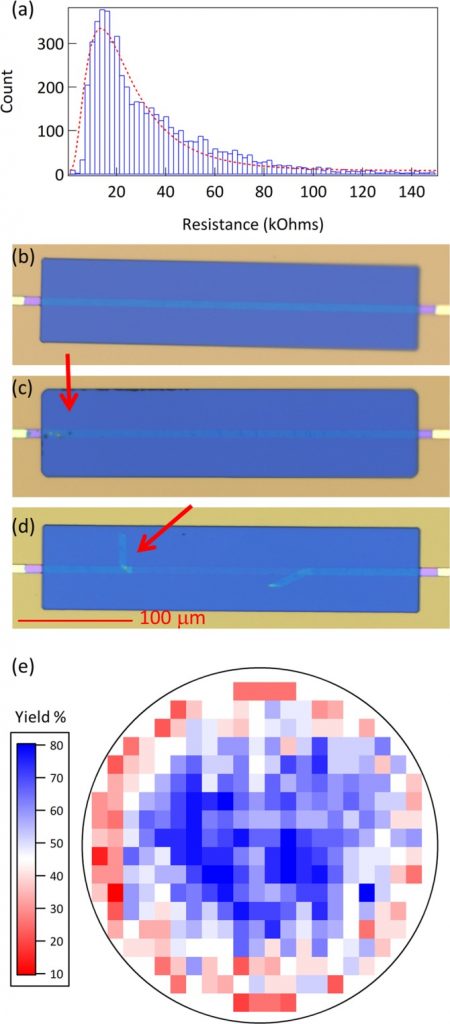
Adeno-associated virus (AAV) vectors currently represent the most attractive platform for viral gene therapy and are also valuable research tools to study gene function or establish disease models.
Consequently, many academic labs, core facilities, and biotech/pharma companies meanwhile produce AAVs for research and early clinical development. Whereas fast, universal protocols for vector purification (downstream processing) are available, AAV production using adherent HEK-293 cells still requires time-consuming passaging and extensive culture expansion before transfection.
Moreover, most scalable culture platforms require special equipment or extensive method development. To tackle these limitations in upstream processing, this study evaluated frozen high-density cell stocks as a ready-to-seed source of producer cells, and further investigated the multilayered CELLdisc culture system for upscaling.
The results demonstrate equal AAV productivity using frozen cell stock-derived cultures compared to conventionally cultured cells, as well as scalability using CELLdiscs.
Thus, by directly seeding freshly thawed cells into CELLdiscs, AAV production can be easily upscaled and efficiently standardized to low-passage, high-viability cells in a timely flexible manner, potentially dismissing time-consuming routine cell culture work. In conjunction with a further optimized iodixanol protocol, this process enabled supply to a large-animal study with two high-yield AAV2 capsid variant batches (0.6-1.2 × 1015 vector genomes) in as little as 4 weeks.