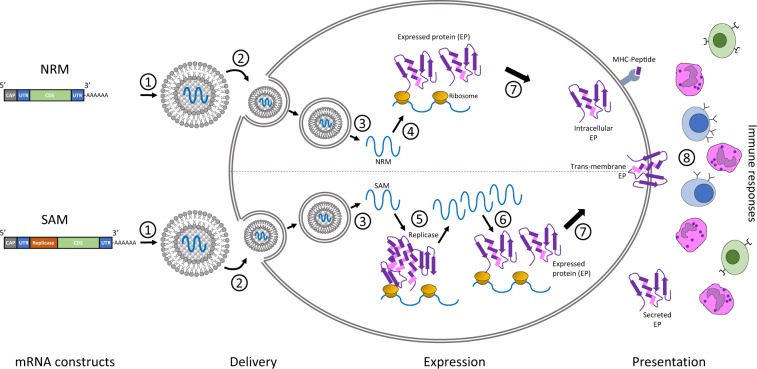
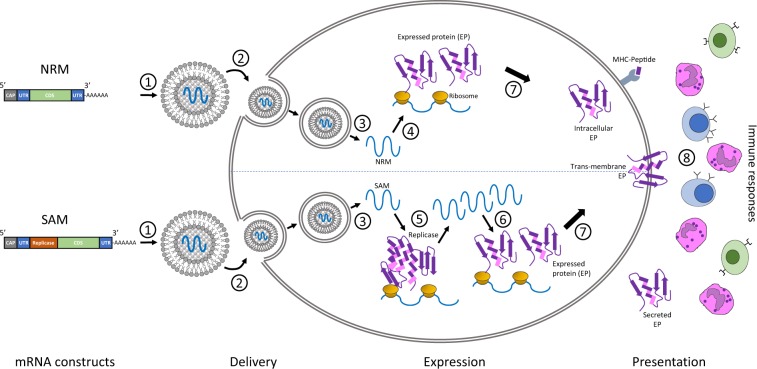
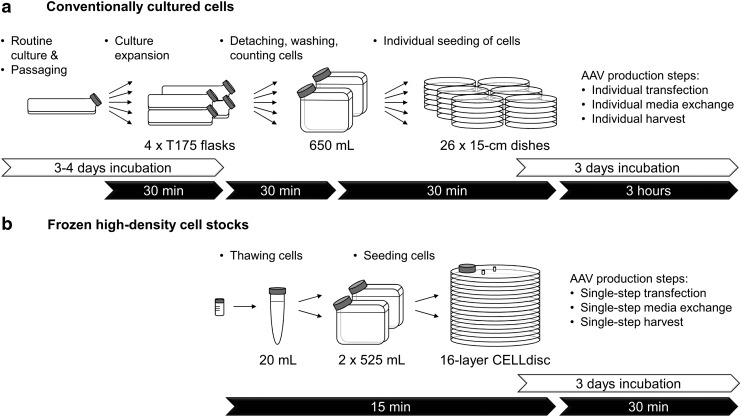
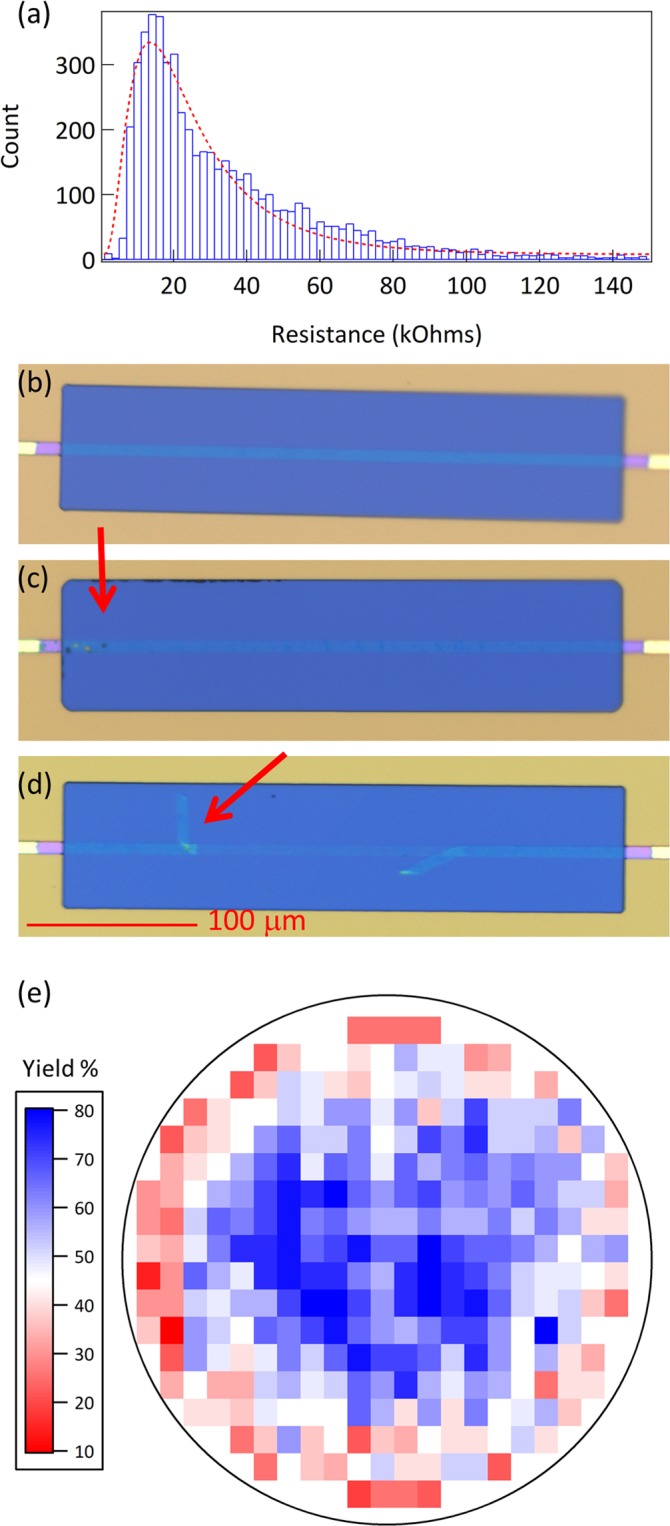
With a projected inhabitants of 10 billion by 2050, an instantaneous precedence for agriculture is to realize elevated crop yields in a sustainable and cost-effective manner. The idea of utilizing a transgenic method was realized within the mid-1990s with the business introduction of genetically modified (GM) crops.
By 2010, the worldwide worth of the seed alone was US $11.2 billion, with business biotech maize, soya bean grain and cotton valued at roughly US $150 billion.
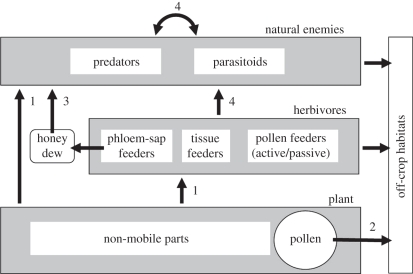
Lately, it has turn into evident that insect-resistant crops expressing δ-endotoxin genes from Bacillus thuringiensis have made a big useful affect on international agriculture, not least when it comes to pest discount and improved high quality.
Nonetheless, due to the potential for pest populations to evolve resistance, and owing to lack of efficient management of homopteran pests, various methods are being developed. A few of these are based mostly on Bacillus spp. or different insect pathogens, whereas others are based mostly on using plant- and animal-derived genes.
Nonetheless, if such approaches are to play a helpful position in crop safety, it’s fascinating that they don’t have a unfavourable affect on useful organisms at larger trophic ranges thus affecting the functioning of the agro-ecosystem. This broadly held concern over the ecological impacts of GM crops has led to the intensive examination of the potential results of a spread of transgene proteins on non-target and useful bugs.
The findings so far with respect to each business and experimental GM crops expressing anti-insect genes are mentioned right here, with explicit emphasis on insect predators and parasitoids.
Methods for the evaluation of protein aggregates in pharmaceutical biotech product growth.
Inside the European Immunogenicity Platform (EIP) ( http://www.e-i-p.eu ), the Protein Characterization Subcommittee (EIP-PCS) has been established to debate and trade expertise of protein characterization in relation to undesirable immunogenicity.
On this commentary, we, as representatives of EIP-PCS, assessment the present state of strategies for evaluation of protein aggregates. Furthermore, we elaborate on why these strategies needs to be used throughout product growth and make suggestions to the biotech neighborhood with regard to methods for his or her software throughout the growth of protein therapeutics.